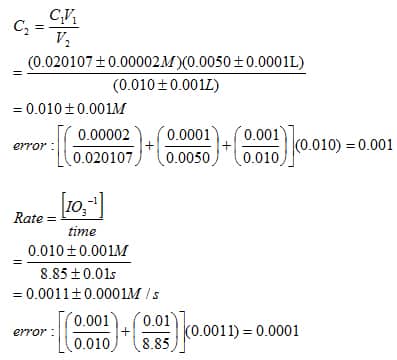
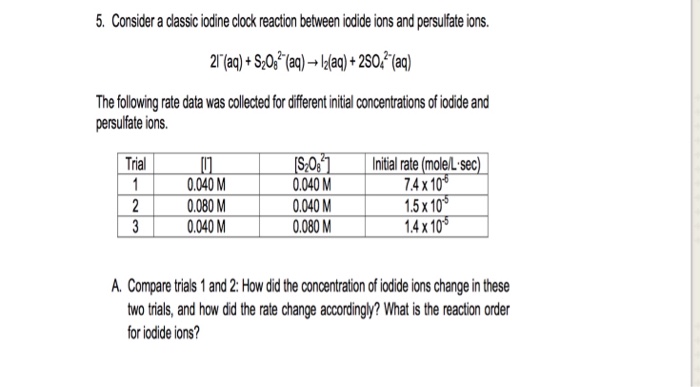
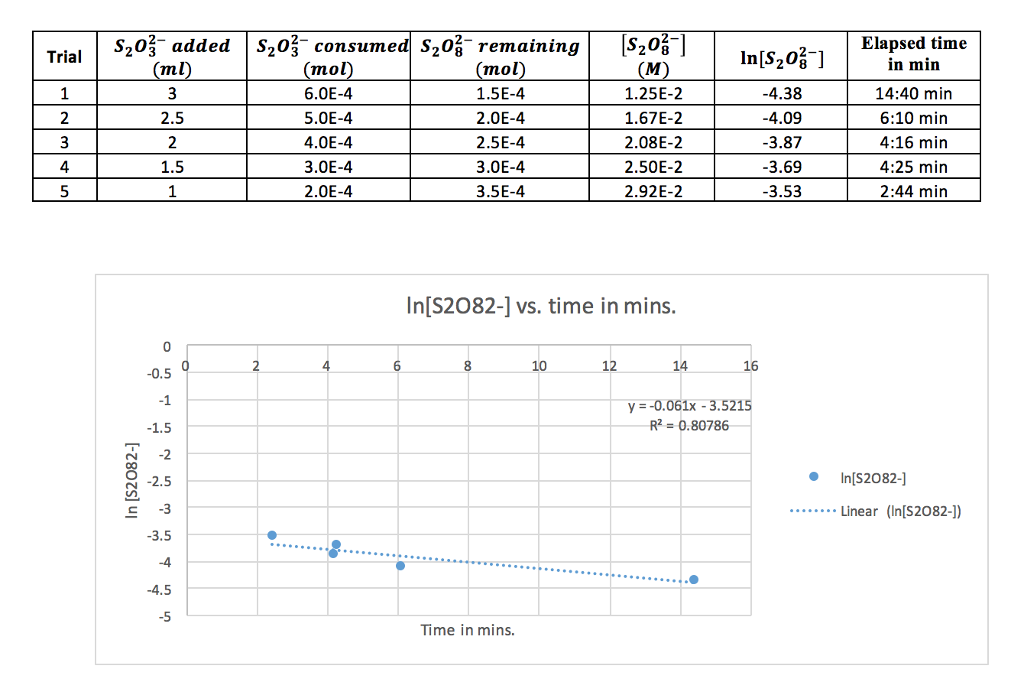
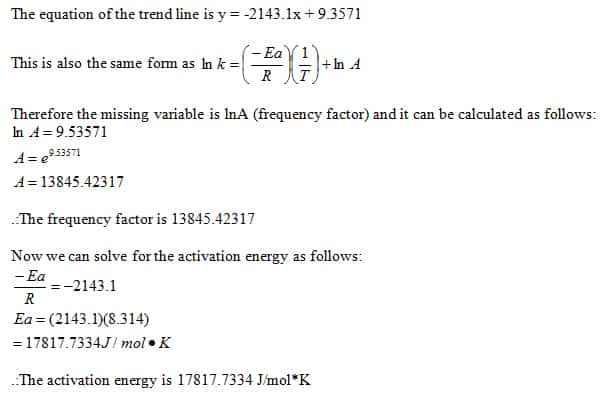SOLVED: 3. Write the overall rate law for the iodine clock reaction below. What is the overall rate order of the main reaction? 5. Hydrogen gas reduces NO to N2 according to

OneClass: In the Rate of an Iodine Clock Reaction experiment, the following data were collected by a ...

SOLVED: Proposed mechanism for the iodine clock reaction is shown below: Iodine Clock Reaction Mechanism IO3 + HSO3 â†' S0 + HIO2 (k = 2.95 x 10^-1 M^-1s^-1) HIO2 + I- +
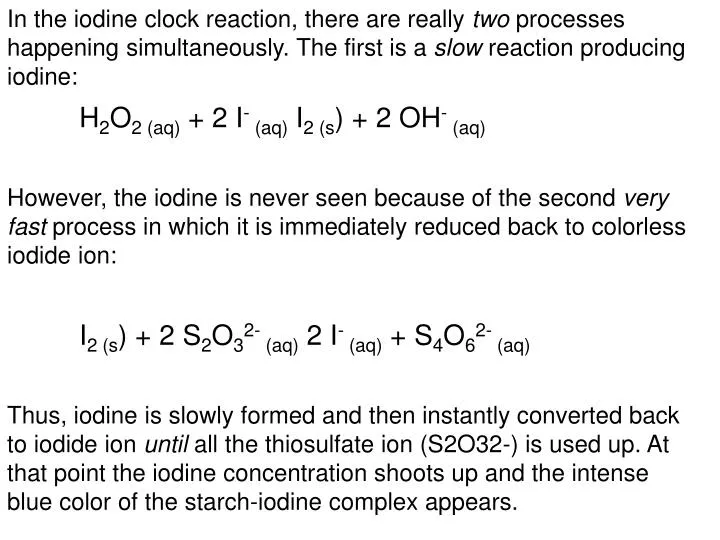
PPT - In the iodine clock reaction, there are really two processes happening simultaneously. The first is a slow reaction PowerPoint Presentation - ID:39733
![SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order](https://cdn.numerade.com/ask_previews/494c958b-2794-4a7f-861f-022f5f445939_large.jpg)
SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order

The general reaction scheme for an iodine clock reaction: (red) species... | Download Scientific Diagram
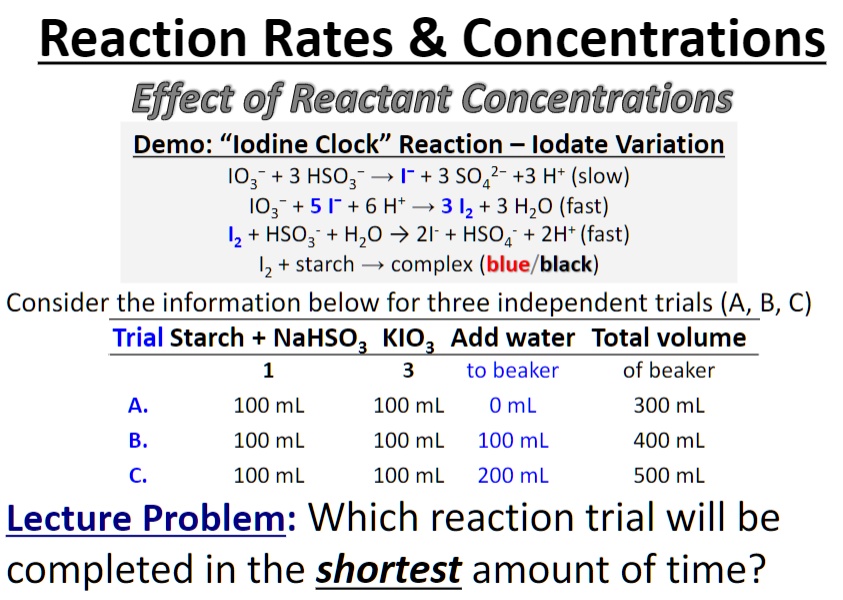



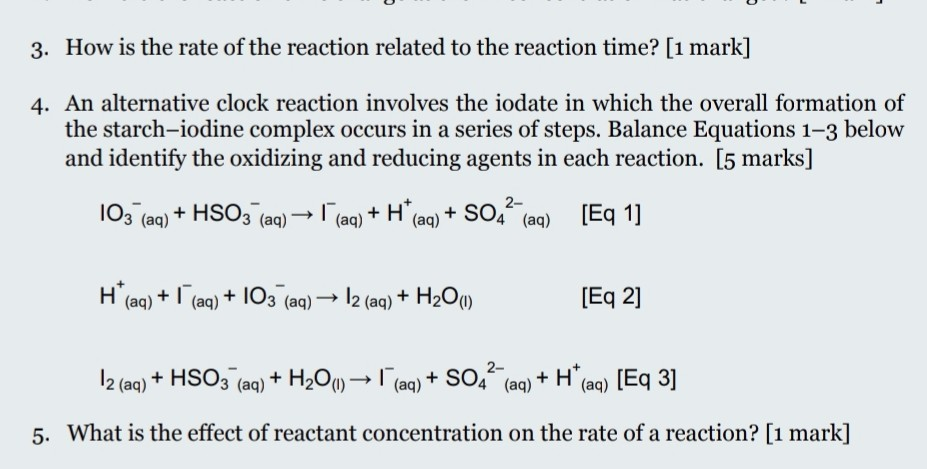
![Solved The iodine clock reaction Test [KIO3] (M) [NaHSO3] | Chegg.com Solved The iodine clock reaction Test [KIO3] (M) [NaHSO3] | Chegg.com](https://media.cheggcdn.com/media/4ea/4ea146b7-b7f4-4915-88de-6a4373a115e2/phpLZvxWR)